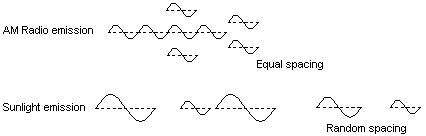Energy Science Made Simple
|
CONTENTS 32pg 171K 32fig 1. Introduction 2. Modern Energy Science 3. Defining energy 4. Theories of what fills the universe 5. Reasons for a Theory of Everything 6. Space continuum, Force fields, Wavicles, Theory of Everything 7. Observer and absolute systems 8. Space continuum, Force fields, Wavicles 9. Wavicles vs particles and waves 10. External energy vs internal energy 11. First and second laws of energy 12. Second-law evaluations 13. Forms of energy 14. Units 15. Temperature of internal energy 16. Types of mass and energy 17. Momentum, impulse, acceleration, forces 18. Energy vs work and heat 19. Caloric energy 20. Helmholtz energy 21. The carnot ratio and helmholtz ratio 22. Helmholtz ratio vs entropy 23. Reversible and irreversible processes 24. Energy transformation 25. Efficiency terms 26. Is perpetual motion possible? 27. Hydro-power 28. Solar energy conversion 29. Gas turbine 30. Electrochemical fuel cells 31. Refrigerator 32. Food calories to muscle power 33. Energy science history 34. Symbols 35. Notes 36. References 37. Revision history >>>Copyright 2023Mar05 by Ben Wiens...energy scientist
1. INTRODUCTION
Fig 1 Not many people understand present energy concepts such as entropy
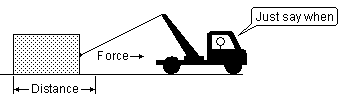
2. MODERN ENERGY SCIENCE Fig 2 Energy is the ability to create a force over a distance some time in the future
Energy is not that old a word, Thomas Young proposed it in 1807 [2]. Initially energy was thought of as the ability to do macroscopic work like the work the kinetic energy of a cannonball can do on impact or the work that the potential energy of a hoisted block of granite can do on falling. Work was defined as...a force acting over a distance. Later in 1830 Joule said that this energy or ability to do macroscopic work was also contained within the molecules. He suggested that the molecules contained forms of microscopic energy just like that contained in the moving cannonball and the hoisted block of granite. In 1905 Einstein suggested that matter such as the molecules and atoms are really very large collections of energy particles. As well there existed energy particles such as the photon that transferred energy between different types of matter. Under some conditions some of these energy particles could be liberated from the atoms in different forms such as light and heat. When we feel the weight of an apple in our hand we are really feeling the weight of the energy particles in the apple. How could energy be defined then according to modern science [4]? Energy...the ability to create a force over a distance in the universe from the very smallest event within the tiniest of particles to the very largest between celestial objects in the universe. Work...a force acting over a distance in the universe from the very smallest event within the tiniest of particles to the very largest between celestial objects in the universe. Energy then is the ability to do work some time in the future. It is stored work potential. Work is the actual act of the force acting over a distance.
4. THEORIES OF WHAT FILLS THE UNIVERSE |
| Symbol | Description |
| LET | Lorentz aether theory...the latest aether theory before special relativity |
| SR | Einstein Special Relativity theory...all motion is relative in the universe |
| QT | Einstein Quantum Theory...light is emitted as wavelike particles |
| GR | Einstein General Relativity theory...gravity causes space to be curved |
| QM | Quantum Mechanics...all energy is composed of particles and probabilities of location |
| UFT | Unified Field Theory...attempt at finding common entity for all four forces |
| QFT | Quantum Field Theory...forces are the result of different types of force particles |
| GUT | Grand Unified Theory...unification of strong, weak, electromagnetic interactions |
| TUT | Totally Unified Theory...explain working of universe down to single particle |
| TOE | Theory of Everything...try to explain working of the universe as the biggest picture |
|
Fig 3 Major theories of how the universe behaves
5. REASONS FOR A THEORY OF EVERYTHING
Fig 4 Einstein rarely mentioned details of the space continuum that energy travels in
One of the major things that is missing from most of the theories is, what fills space. For example Einstein's Special Relativity theory renounced the aether as the medium that light traveled in. Many people to this day think that Einstein's Special Relativity theory proposed that light and matter traveled in space that was totally void. Though it was not mentioned in the theory, Einstein later cleared up this matter by saying that "this rigid four-dimensional space of the Special theory of Relativity is to some extent a four-dimensional analogue of H.A. Lorentz's rigid three-dimensional aether" [1]. So Einstein's space-time in his Special Relativity theory can be thought of as a type of aether. Einstein didn't like to use the word aether however and preferred the word space-time. This was because the aether was commonly thought to be made of a type of gas that was made of matter. His space-time was a type of structure of space which was not made of matter. Unfortunately his explanations of what space-time really is were often not mentioned in his books.
6. SPACE CONTINUUM, FORCE FIELDS, WAVICLES, THEORY OF EVERYTHING
Fig 5 The energy in each photon is related to its wavicle-frequency relative to the space continuum
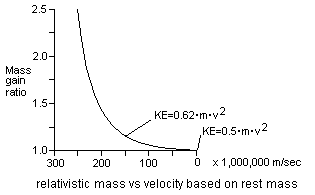
Most energy is composed of vibrating wavicles. When energy wavicles travel at high speed relative to the space continuum there is a Doppler [6] type effect that occurs. In Fig 5 there are two diagrams, one with the star and planet at rest relative to the fixed space continuum, and a second with the star and planet moving relative to the space continuum. The star, planet, and the human observer are made of matter that are really just a collection of vibrating wavicles. When matter is forced to increase its speed relative to the space continuum, the vibrating matter wavicles are forced to vibrate quicker as well. This is the reason matter gains a large amount of relativistic mass [3] when it travels near the speed of light. Electrons have been accelerated to near the speed of light in particle accelerators. Some of these electrons have more than 1,000 times as much relativistic mass than their normal rest mass.
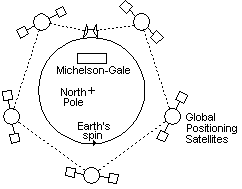
7. OBSERVER AND ABSOLUTE SYSTEMS
Fig 6 The coriolis effect can be explained as the earth spinning in a fixed space continuum
Einstein popularized a certain relative system of measurement which could be called the Observer measurement system. Because of the Doppler effect that occurs when energy wavicles travel in the universe, and because of the speed of light, measuring tools, clocks, and mass all change as an observer moves relative to the space continuum. An observer...is considered to be a human or an instrument that is measuring the event. So it is impossible to get an absolute measurement of distance, time, and mass in relation to the space continuum. So in the Observer system, time is based on clocks the observer has which vary their speed depending how fast they travel relative to the space continuum. Distances in the universe are based on how quickly a ray of light can travel from the distant event. Measurements of mass become only comparisons with other frames of reference.
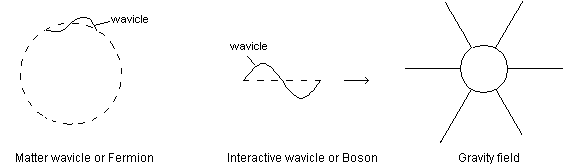
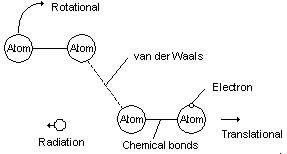
8. SPACE CONTINUUM, FORCE FIELDS, WAVICLES
Fig 7 Matter wavicles are really just collections of interaction wavicles
Presently force fields and energy wavicles are not very well understood in science [5]. The biggest dilemma is that currently most scientists think that forces are produced by the exchange of interactive wavicles such as photons. In this view it is imagined that the magnetic field is actually a flow of virtual photons emanating from one end of the magnet and being returned in the other. However it is also a well known fact that different types of blackbodies absorb every type of real photon that is emitted. Surely then a blackbody placed in the path of a stationary magnetic field would absorb the virtual photons that were emitted and quickly heat up. At least the magnet would absorb the photons if the virtual photons were anything like a real photon. If virtual photons aren't at all like real photons then they are a completely different kind of entity than real photons with completely different properties. In actual fact force fields do not behave in any kind of way that would suggest that they are made of particles at all.
9. WAVICLES vs PARTICLES AND WAVES
Fig 8 We see color based on the frequency of individual wavicles and not on spacing between wavicles
It is understood by most modern scientists that the traveling vibration of energy wavicles is not like ordinary water waves or sound waves. Still textbooks continue to use the standard water wave model to represent energy wavicles. Energy wavicles in fact travel in a completely different way. If a single water wave is generated in the center of a calm body of water, it travels in a spherical pattern from the source. The further away from the source, the weaker the wave becomes. A boat lying far from the source of the wave would experience only part of the energy of the wave as it passes by the boat. This does not happen with the energy wavicle. A single energy pulse can travel for years in space without spreading out if it does not come in contact with matter wavicles. When it is finally absorbed, the entire wavicle will be absorbed into one atom. Not only does the pulse not spread out sideways, the vibration is confined into a small zone as it moves forward. |
|
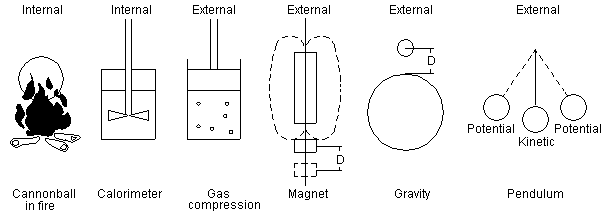
10. EXTERNAL ENERGY vs INTERNAL ENERGY
Fig 9 Internal energy and external energy forms
If a cannonball, as shown in Fig 9, is heated in a fire it will become hotter but it will not take off out of the fire and leap into space. Why not? The energy that was added was thermal energy. The energy added increases the velocity of the molecules, however the molecules are traveling in all different directions and this does not result in any forward movement of the collection of molecules. This type of microscopic energy is called multidirectional energy. If energy was added to the cannonball by a charge of gunpowder in a closed cannon, the expanding hot gases would be made more directional and so the cannonball would come flying out of the bore of the cannon. The cannonball flying out of a cannon would contain both multidirectional and directional forms of energy. The cannonball however does not contain some molecules that are only traveling forward and some that are multidirectional. The two types of energy are superimposed on one another. In a cannonball flying through the air, though the molecules may be traveling in all directions, the net direction of all of them is forward. It is difficult to understand how directional energy could be stored in the same wavicles as multidirectional energy. Why would the two forms not get mixed up? Why would the directional part not slowly become multidirectional? This does not occur because the energy wavicles do not change direction until acted on by some outside force. There would need to be a directional force canceling out the directional part. Even though the energy wavicles are moving in all directions, the wavicles energy and direction is still perfectly accounted for in the process. If there is a mean direction in the sum of all the multidirectional energy, this will be conserved.
11. FIRST AND SECOND LAWS OF ENERGY
12. SECOND LAW EVALUATIONS
Fig 10 Einstein energy can logically be divided into different forms
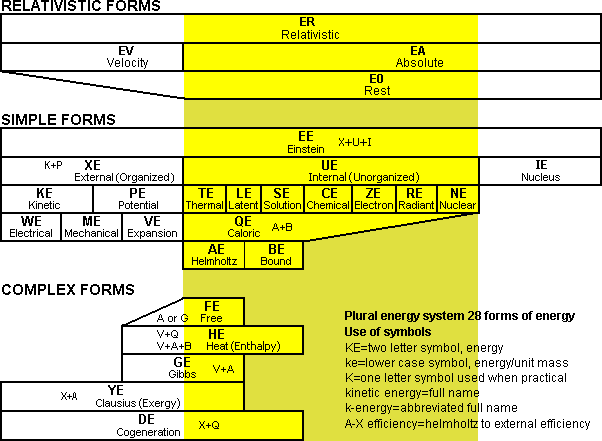
It is beneficial to have a proper naming system that covers all the basic types of different energy in the universe. This is because it is often difficult or impossible to convert certain types of energy into other different forms. The system of energy proposed in this booklet is based on the plural energy naming system where all the different types of energy are two word forms such as nuclear energy. The basis of this two word naming system is borrowed from chemistry. Many scientists over time have tried to establish single word names like enthalpy, essergy and anergy for the different forms of energy but this is not necessarily desirable as few people would recognize them as forms of energy. It is also confusing for some forms of energy to be two words such as internal energy while others are not such as enthalpy. As well there are not enough different names that sound similar to energy that can be used when many forms are used. To be consistent, these single word energy names were converted into two word names in the plural energy system.
Fig 11 Popular 28 forms of energy in the plural energy system shown in a bar chart
There are another seven complex forms of energy shown in the third chart of Fig 11. Substances usually contain mixtures of external energy and internal energy. Scientists have developed many terms for combinations of these two types of energy. The reason these combinations are used is that it is often too much work to separate an internal or external energy addition.
Fig 12 Places where energy is stored in internal energy
14. UNITS 100XE=100QE=30AE + 70BE
In science, the metric system is the preferred system of units around the world. In the United States, engineers often use the old English system of units, which is now named the American system. In this system the ft-lb is the unit of external energy and the BTU is the unit of internal energy. The older metric system also had such dual units. The joule or newton-meter was considered the unit of external energy and the calorie was the unit for internal energy. When doing calculations for energy conversion devices such as gas turbines, it is too cumbersome to use dual units for energy. Usually one or the other was picked. This is why presently the more preferred SI metric system uses one unit of energy. |
|
15. TEMPERATURE OF INTERNAL ENERGY
Fig 13 Internal energy has varying amounts of energy per wavicle
Energy is a two dimensional property of force times distance potential. The previously mentioned First Law of Energy says nothing about the ability of different energy wavicles to generate a certain amount of force. As was already mentioned, all forms of external energy can be theoretically converted entirely into other forms of external energy. For example a block and tackle can be used to multiply the force that can be delivered. By this means someone can pull with a 100 kg force and raise a block of 2000 kg. Internal energy cannot be converted in this way. It is like the tow truck shown in Fig 13. Imagine that the tow truck has enough gas in the tank to provide 100 units of energy. Could it be assumed that the tow truck can pull a train up a very steep grade with a total of 10 units of force but only for a very short distance of 10 units? Not really! The tow truck may not have the capacity to provide this amount of force.
16. TYPES OF MASS AND ENERGY
MA = absolute mass, kg
EA = absolute energy, joules
v = velocity of mass in m/sec relative to space continuum
When a cannonball is stationary relative to the space continuum, it contains an amount of absolute mass. When a cannonball is stationary relative to an observer, it contains an amount of rest mass. When the cannonball is accelerated through the air, it's total relativistic mass increases. In physics it is usual to only calculate the increase in the relativistic energy which is the increase of it's velocity energy.
Because the cannonball has gained energy, it has also gained mass. It is possible to calculate this mass through a type of relativistic Doppler formulae, which is called the FitzGerald relationship, as shown in Eq (2), which equates the velocity of the rest mass to the new larger relativistic mass of the cannonball. The gain in velocity mass can be calculated with Eq (3) which subtracts out the rest mass. If this mass is plugged into the well know Einstein Eq (4), it will be found that the energy calculated will be identical with what is calculated with Eq (1). Note that Eq (4) does not look like the usual Einstein equation because it uses the more exact symbols for energy and mass.
Fig 14 The formulae to calculate the velocity energy changes with speed
When a nuclear fission explosion occurs such as in an atomic bomb, the original collection of matter wavicles of the uranium are rearranged into a new collection of matter wavicles. There is a small amount of mass left over that cannot be utilized in the formation of the new collection of wavicles. This small amount of mass results in a great deal of energy being liberated, largely in the form of super high energy photons. The amount of energy liberated can be calculated with a variation of Eq (4) using absolute mass and absolute energy. Because both matter and photons have both relativistic mass and relativistic energy, it is not proper to say that mass is converted into energy in the system suggested here. It is better to say that a certain amount of matter was converted into other forms of matter and released excess energy in the process . 17. MOMENTUM, IMPULSE, ACCELERATION, FORCES
Fig 15 Momentum is different than energy
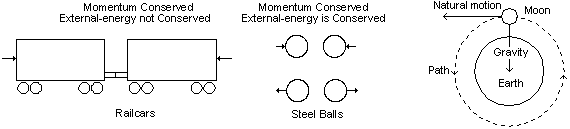
As was mentioned in Chapter 6, there is a system that energy travels in composed of the space continuum. A key characteristic of the space continuum is that energy travels in straight lines if there is no interference from other energy wavicles or force fields. Why it does so is not understood presently. Besides wanting to travel in a straight line, another property of the space continuum is that energy wants to travel in the same direction and at the same speed. Both of these properties can be considered to be the momentum of the energy. Energy wavicles have momentum because if they change in velocity relative to the space continuum, the Doppler effect requires that they change their energy content. When energy is not available or cannot be disposed of, they cannot change in speed. (Eq 5) Momentum=MR·v
MR = relativistic mass, kg
18. ENERGY VS WORK AND HEAT
Fig 16 Caloric energy is the amount of internal energy that would flow from one system to another
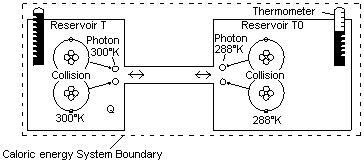
In the gas expansion examined in Chapter 10 there is a type of flow of the multidirectional energy of the molecules from a small space into a larger space. Conversions from one form of external energy to another form of external energy such as this can occur in a single reservoir of energy. Converting internal energy such as thermal energy to external energy is much more difficult and a flow between two reservoirs is required. A reservoir...is considered to be a source of internal energy in equilibrium or at the same uniform temperature. In this booklet the flow between two reservoirs is called caloric energy. This term is not used in present textbooks. Presently it is said that the difference in total internal energy of two systems results in a flow, but it is easier to deal with a single energy rather than a difference of two energies. Carnot described this flow of internal energy to be similar to a waterfall. This waterfall though is not external energy, it is rather a waterfall of internal energy. |
|
20. HELMHOLTZ ENERGY
Fig 17 The carnot ratio is the amount of helmholtz energy in an amount of caloric energy
The maximum amount of external energy that can be extracted from two reservoirs of truly unorganized internal energy is predictable. The model shown in Fig 17 can be used to analyze such interactions. In classical thermodynamics such a model for thermal energy was based on the molecular kinetic theory, which depicts molecules colliding against each other and gases expanding. The model in Fig 17 is based rather on the quantum model where all forces between matter wavicles are the result of electromagnetic force fields. When two atoms collide they do not merely bounce off each other, the impact of the collision is absorbed temporarily by the atom's electron, which gains energy. This increased energy does not result in a stable orbit for the electron, it wants to shed this energy as quickly as possible. It appears that this is because the electron wavicle has shifted into an orbit where its vibrations are no longer even divisions of the orbital path. Sometimes the atom immediately reabsorbs the energy from the electron into the translational field, which propels the wavicle in the opposite direction. At other times a real photon is emitted, which is radiated away from the atom in the form of radiant energy. Such photons are often quickly absorbed in neighboring atoms, but if the atoms are next to a vacuum such as for example outer space, they could travel for thousands of years with little change.
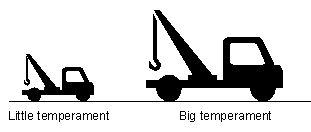
21. THE CARNOT RATIO AND HELMHOLTZ RATIO
Fig 18 The carnot ratio plotted on a graph shows the changing conversion efficiency during a process The example just mentioned in Chapter 20 however only involves a single photon being converted. Real life energy conversion processes involve millions of photons. Also the temperament of Reservoir T may vary during the process. The carnot ratio Cr...can be used to represent the external energy that could be extracted from a flow of caloric energy at an instantaneous moment such as when a single photon is converted [21]. It can be calculated with Eq (6). The helmholtz ratio Ar on the other hand...represents the external energy that could be extracted during the entire flow of caloric energy. (Eq 6) Cr=1-T0/T
T0=constant ambient temperature flow of internal energy, infinitely large reservoir
The carnot ratio can be plotted on a graph as shown in Fig 18. Based on a fixed ambient temperament T0, a single curve can be drawn. This is a useful tool for analyzing any conversion process because it shows what the changing conversion efficiency will be during the process. Such a curve merely represents the changing amount of external energy that could be extracted from an amount of internal energy. Though there is only one curve, it can be used in different ways. Later in Chapter 23 reversible and irreversible processes will be discussed. In a reversible process, the temperament can rise or fall along the curved line without any loss of the external energy that was put into the process. The helmholtz energy stored in the system will always equal the amount of external energy that was added or can be removed. In an irreversible process, there will be a difference in the amount of external energy added vs the amount of helmholtz energy stored. The curve represents the amount of helmholtz energy stored in the system and this can then be compared to the larger amount of external energy that was added to the system.
(Eq 7) Ar=1-T0/T (T is temperature) For variable temperature processes of constant specific heat such as perfect gases Eq (8) can be used [22]. Air is quite close to a perfect gas at temperatures between 250°K to 1000°K and so this equation is still often used. Even at higher temperatures compensating factors can often be used, which allows this equation to still model a process.
(Eq 8) Ar=1-ln(T2/T1)·T0/(T2-T1) For variable temperature processes of variable specific heat such as steam processes, entropy is often used with Eq (9 plus 10). Entropy will be covered in the next chapter. There is however a way to avoid using entropy entirely by using complex formulas and calculating the helmholtz ratio directly.
(Eq 9) BE=T0·S (S is entropy in this case) The helmholtz ratio can be calculated for any type of internal energy. It can be thermal, chemical, nuclear energy etc. When the helmholtz ratio has been calculated it can be used in a variety of equations such as Eq (11 and 12). From these basic equations all the other forms of energy shown in Fig 11 can be calculated.
(Eq 11) Ar=AE/QE
22. HELMHOLTZ RATIO VS ENTROPY
Fig 19 The helmholtz ratio method vs entropy in gas compression process
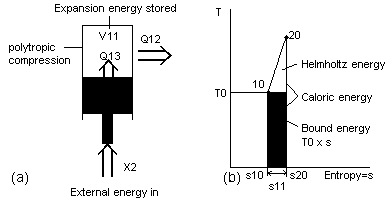
In present textbooks the amount of external energy that could be obtained from a flow of caloric energy is always calculated using a term called entropy. Entropy often is confusing to people learning energy [23]. This is because entropy is often implied as being a type of energy. It is not. Previously the unit of energy called bound energy was mentioned. Entropy...is a number that can be multiplied by the ambient temperature of Reservoir T0 to obtain this same bound energy. Entropy is used in processes where the properties of matter are not uniform with temperature and pressure and so cannot be easily calculated with formulas. Because of this, tables are used that list entropy at these different conditions. Instead of estimating the bound energy from a graph such as Fig 18 or calculating it from Eq (8), the values of entropy corresponding to the conditions at T2 and T1 are looked up. The difference of these two figures represents the changing entropy during the process. The change in the bound energy can then be calculated. Only one table is required with entropy even though calculations are done at different ambient temperatures. It is possible to use a property such as entropy because the Reservoir T0 does not change in temperature during the conversion. (Eq 13) AE=QE-(T0·S) where S is the possible change in entropy of two states of the system such as s11 of Fig 19
The numbers used in entropy have little meaning by themselves, while the helmholtz ratio can be a useful figure by itself. It is a ratio or a percentage. If the helmholtz ratio of a certain system is 0.50 or 50%, it can immediately be visualized that a maximum of 50% of the caloric energy could be converted to external energy. Because most calculations are generally done at a set ambient temperature of 15°C, the helmholtz energy does not need to be recalculated very often for each process.
23. REVERSIBLE AND IRREVERSIBLE PROCESSES
Fig 20 Model demonstrating energy conversion and the carnot ratio
It is often said that the multi-directional motion of the molecules is more disordered than the directional energy of external energy and this is the reason it cannot be entirely converted into external energy. The concept of entropy, which was discussed in detail in Chapter 22, is largely understood in terms of disorder. The gas expansion examined in Chapter 10 is multi-directional energy however and it is considered to be a form of external energy. This is proof that multidirectional energy can be "straightened" entirely into external energy without losses in some cases. So it is difficult to relate order or disorder with a systems ability to be converted entirely in external energy. Because of this it is better to relate to the concept of reversible and irreversible processes. A reversible process...is a process which once having taken place, can be reversed back to the original conditions by some sort of process and in doing so leave no change in either the system or the surroundings. An irreversible process...is a process which once having taken place, cannot be reversed back to the original conditions by some sort of process and in doing so leave no change in either the system or the surroundings. It is really changes in the total order in the universe that are important. External energy can be perfectly stored in many systems that are disorderly, but if there is no change in the degree of disorder in the universe, then the system is reversible. This is true as well when working with entropy, the total entropy of a system is usually unimportant, it is rather the change in entropy that is important.
Fig 21 Low amount of caloric energy made into high amount of caloric energy in an energy transformer
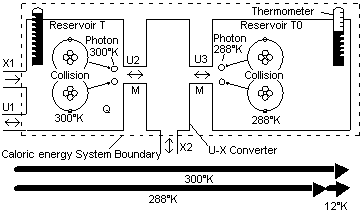
In an electrical transformer, high voltage low current power can be efficiently converted into low voltage high current power. Internal energy of a high temperature but low caloric energy content can also be converted into low temperature but high caloric energy content. Such a transformation is simplistically modeled in Fig 21 by connecting two system diagrams from Fig 17 together. In this diagram it can be shown how one form of internal energy with a high temperature such as methane fuel could be converted into another type of internal energy of a lower temperature but higher internal energy. Methane of 891 kJ enters Reservoir T3. This chemical energy has a high mean chemical temperature of about 3,500°K. X-energy of 819 kJ is extracted and transferred to another caloric energy system where 10,254 kJ of internal energy is created at 313°K thermal temperature. About 11.6 times as much caloric energy was created than was originally put in. The internal energy that is transformed can be thermal energy, latent energy, chemical energy etc. In the plural energy system different types of energy can be created but the total einstein energy of course is the same. |
|
25. EFFICIENCY TERMS
Fig 22 Efficiency of converting some forms of energy to X-energy in large gas turbines
Presently it is said that certain steam engines are 30% efficiency in converting thermal energy into external energy. In the system proposed in this booklet however efficiency when used by itself is a word that is used only when converting similar types of energy. Because the First Law of Energy applies to all processes, they will all be 100% efficient in converting einstein energy to einstein energy. In a sense, internal energy and external energy are like two completely different energy entities. Because a larger amount of caloric energy is turned into a smaller amount of external energy or vis versa in a perfectly reversible process, this shouldn't be called an efficiency either. In the present system a refrigerator can have an efficiency higher than 100%, which does not sound right in this case and so a term of coefficient of performance is used.
26. IS PERPETUAL MOTION POSSIBLE?
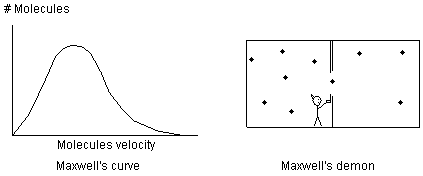
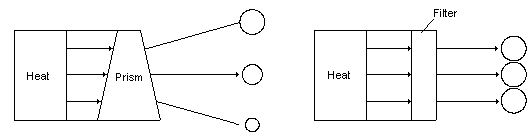
Fig 23 Maxwell joked about a demon that would sort hot and cold molecules A more modern perpetual motion concept is visualized by separating light with prisms or filters. Why try and separate hot and cold molecules when you can separate hot and cold photons that are emitted by the hot and cold molecules? Instead of trying to separate a heavy molecule with only a tiny bit of velocity energy contained it its motion, the photon represents raw, pure velocity energy. It is known that white light can be separated with a prism into photons of different energies or temperature. It is also known that at room temperature many substances are emitting photons of various energies of infrared light. Would it not be possible to separate these photons into different colors with a prism or a filter as shown in Fig 24 and then use the high and low temperature light to operate a heat engine? Apparently not. So far all the mechanisms that have been dreamed up all comply with the rule of equilibrium [24] and don't result in any production of external energy.
Fig 24 Using prisms and filters as Maxwell's demon
Some have suggested that a heat pump or refrigerator could be used to create more energy than was put in. A heat pump or refrigerator cannot create any more helmholtz energy in an amount of caloric energy than the external energy put in. This helmholtz energy is the external energy stored in the heat.
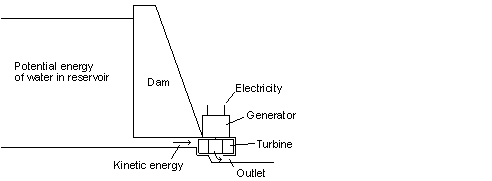
Fig 25 The water stored behind the dam is a form of external energy
In the hydro electric dam, the water stored at a higher elevation is a source of potential energy. It is converted to kinetic energy in the turbines and then to electrical energy. These are all forms of external energy. As with the pendulum example in Fig 9, different forms of external energy can theoretically be entirely converted to other forms of external energy. While not every conversion is as simple as in the case of the pendulum, conversion is easier than when converting internal energy to external energy. Generally more than 90% of the potential energy of the water can be converted into electrical energy, which means that the P-W efficiency is 90%. It is also very easy to start and stop any one of the turbines and there is little loss in doing so. Hydro electric dams are perfect for supplying peaking power during periods of the day when more electric power is used than average. The potential energy that is not converted to electric energy is converted into thermal energy. This is done at such low temperatures that almost no helmholtz energy is left in the thermal energy.
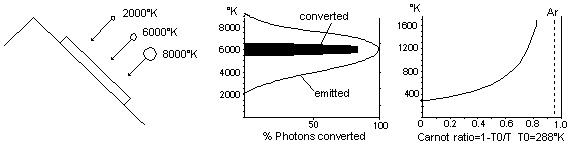
Fig 26 Solar cells presently cannot utilize all the energy of sunlight
The light from the sun has an average temperature of about 6,300°K. The carnot ratio diagram of Fig 26 shows that the helmholtz ratio of sunlight is about 95%. This means that theoretically it should be possible to convert 95% of the radiant energy to electricity. In actual practice, present day common solar cells only convert about 11% of the radiant energy into electricity at most. This means that 89% of the remaining sunlight is converted into thermal energy. Solar cells are actually sheets of semiconductor material. When the sunlight strikes the semiconductor an electrical potential is created by dislodging electrons from the impact of the photons. Sunlight however is made of photons that contain different amounts of photon energy and different wavicle-frequency. The semiconductor cannot easily be tuned to receive all types efficiently. This means that some photons will not be converted at all because they have too little photon energy and some photons will only have a part of their energy converted to electricity because they have too much photon energy as shown in Fig 26. Does the temperature of the photons change on cloudy days? Only slightly. When a magnifying glass is used to concentrate light on a small area a hole can be burned through the paper. The temperature of the bright spot of light is the same as the light before the magnifying glass but because the light was spread out so much, the heat generated was dissipated too quickly for its temperature to rise. The brightness of the light changed, but the average photon energy of the photons did not change.
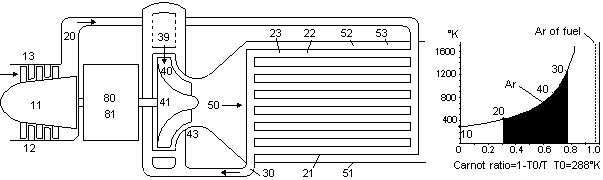
Fig 27 Much of the temperature of the fuel in a heat engine is lost in the combustion chamber
Chemical energy or fuel represents a source of energy that can easily be stored, transported or piped to where it is needed. Fuel represents the most energy that can be obtained from a kilogram of substance next to using nuclear energy. Unfortunately fuel is internal energy. This type of energy is very hard to convert to external energy, which is the type that is needed for producing electricity or propelling vehicles. Engines such as steam engines and diesel engines must first convert the temperature of the chemical energy to thermal energy and then expand the hot gases produced to create external energy. A typical fuel such as jet fuel has a maximum chemical temperature of up to 20,000°K. This can represent a helmholtz ratio of up to 99%. There are two reasons why such a high temperature cannot be used in an engine. First, the products of the combustion process would dissociate at such a high temperature and absorb the energy, thereby lowering the final temperature produced. This is called the equilibrium temperature. Secondly no present day engine can properly utilize even the equilibrium temperature without melting or oxidizing the engine parts. Cooling the combustion gases before expansion results in a lower helmholtz ratio of the combustion gases, which means that less external energy could be extracted from the combustion gases in the gas turbine.
|
|
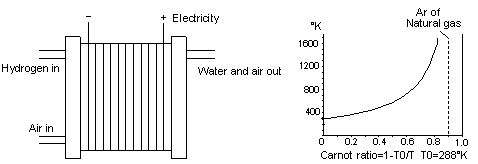
30. ELECTROCHEMICAL FUEL CELLS
Fig 29 A fuel cell can convert the temperature of the fuel directly into electricity
Heat engines are not available that can run hot enough to properly use the chemical temperature of fuels. A fuel cell creates electrical energy, which is a form of external energy, directly from the chemical temperature in fuels without an intermediate conversion into thermal energy. This is possible because the high temperature of the fuel energy was converted into electrical energy through the action of virtual photons as discussed in Chapter 15. In this way it can theoretically convert all the helmholtz energy of the fuel to electrical energy. As shown in Fig 29, natural gas fuel would have a helmholtz ratio of greater than 0.90 or 90%. This means that theoretically it would be possible to convert 90% of the fuel's internal energy into electricity. In actual practice there are a lot of frictional losses in the fuel cell. In a fuel cell, the helmholtz energy in the fuel is converted into ions, these are atoms with less or more the normal amount of electrons. Frictional losses due to movements of these ions, and of the electricity that is created, cause considerable amounts of thermal energy to be produced.
Fig 30 A refrigerator creates more caloric energy than the external energy put in
In a refrigerator more caloric energy can be created than the external energy that was put in. In Chapter 23 we saw that by having two heat reservoirs, it is possible to create a potential flow of heat between them, which is called caloric energy. This caloric energy can be a flow from a hot reservoir to a cold ambient reservoir, or from a cold reservoir to a hotter ambient reservoir. If this caloric energy is created at a very small temperature difference, it does not take much external energy to create a larger amount of caloric energy. Theoretically it would be possible to create 10,000 joules or even more of caloric energy with only 1 joule of external energy. This does not mean that the 9,999 joules was obtained for free. According to the First Law of Energy all the einstein energy must add up. We are removing 9,999 joules of internal energy from one reservoir and pumping it into the other. If this reservoir is the earth there is an almost endless supply. Ar=AE/QE can be reorganized to QE=AE/Ar and assume that XE is turned entirely into AE
QE=1.0 joule/0.07326 Is it possible to create a perpetual motion machine by creating 13.65 joules caloric energy with the 1.0 joule of external energy we put in, then using a heat engine to make more than 1.0 joule external energy back? No, the heat engine would be able to theoretically extract exactly 1.0 joule of external energy from the 13.65 joules of caloric energy that was created.
32. FOOD CALORIES TO MUSCLE POWER
Fig 31 Sugar is converted into muscle power at about 25% A-X efficiency
Human muscles don't convert the chemical energy in foods into heat first as is done in an engine such as a gas turbine. It is often thought that this must mean that muscles are very efficient in converting chemical energy into external energy. Tests have been done with cyclists, and it appears that the body only has a net 25% A-X efficiency in converting food into muscle energy.
Fig 32 The early steam engine converted less than 5% of helmholtz energy in the fuel to external energy
Timeline
34. SYMBOLS
AE = helmholtz energy
Gr = gibbs ratio |
|
35. NOTES [1] Einstein in his book (Einstein 1952) states on p150 "This rigid four-dimensional space of the Special theory of Relativity is to some extent a four-dimensional analogue of H.A. Lorentz's rigid three-dimensional aether". [1a] Einstein admitted in his Special Relativity theory, there is a medium that energy travels . He readily acknowledged this in a book in 1920 (Einstein 1983 p23) where he said "...space is endowed with physical qualities; in this sense, therefore, there exists an ether." [1b] An April 1950 article published in Scientific American (Volume 182, No. 4 pg 3) titled "On the Generalized Theory of Gravitation" Einstein says: "According to general relativity, the concept of space detached from any physical content does not exist. The physical reality of space is represented by a field whose components are continuous functions of four independent variables - the coordinates of space and time." [1c] Einstein in his book (Einstein 1952) states on page 155 that "Space-time does not claim existence on its own, but only as a structural quality of the field". [2] In 1807, the English physician Thomas Young (1773-1829) proposed the word energy for the work potential that is stored within things. It is based on the Greek words like energeia meaning "work within". This term gradually became popular and is now applied to any phenomenon capable of conversion into work (Asimov v1-p94). [3] There are different definitions for mass used in physics. See Chapter 16 [4] We could have rather chosen to define energy as only the directionally oriented or external energy. This in fact would be easier for ordinary people to understand. It is hard to visualize how there can be different types of energy that cannot be completely converted into other forms. When we view the total picture however, it is better that all potential to create a force over a distance be considered as energy. If we do this however, talking about a generic energy is not explicit enough to understand whether it is of any value to us. Hyphenated names like external energy or helmholtz energy must be used to label the type of energy we are talking about. [4a] According to (Ohanian 1985) pg 912 "Thus photons are neither classical particles nor classical waves. They are some new kind of object, unknown to classical physics, with a subtle combination of both wave and particle properties. B. Hoffman has coined the name wavicle for this new kind of object. It is difficult to achieve a clear understanding of the character of a wavicle because these objects are very remote from our everday experience." Einstein it appears did not use the word wavicle. I would however credit him with being the first to understand what they were even though an appropriate name was applied to them later. This is based on a 1909 quote of Einstein (Pais 1982) pg 404 "It is my opinion that the next phase in the development of theoretical physics will bring us a theory of light that can be interpreted as a kind of fusion of the wave and the emission theory...[The] wave structure and [the] quantum structure ...are not to be considered as mutually incompatible...It seems to follow from the Jeans law that we will have to modify our current theories, not to abandon them completely." Though I believe Einstein understood that energy traveled as wavicles, it appears that he was at times quite confused as to their nature. No wonder. Scientists all around him were telling him his idea of a wavelike particle was pretty stupid. This is based on (Pais 1982) pg 383 "Above all, it was obvious to him from the start that grave tensions existed between his principle (light as a particle) and the wave picture of the electromagnetic radiation--tensions which, in his own mind, were resolved neither then nor later...It is curious how often physicists believed that Einstein was ready to retract." [5] Fields are described by some to be similar to a string of beads in (Auyang 1995 p47). [6] That the Doppler effect is the key to all energy is described on p10 of (Calder 1979). This is one of the better and more understandable books on Relativity written because is has background into different issues. [7] It is likely that Einstein based his Relativity theory largely on the ideas of Poincare, a leading French mathematician and physicist. Already in 1895 Poincare was lecturing about his ideas of Relativity which were quite similar to Einstein's 1905 Special Theory of Relativity. Poincare was one of the leading proponents of mathematics by imagination in this time period. He believed in concepts like four-dimensional space. By 1908 however, he changed his mind completely and during the last six years of his life was a convert to the doctrines of observable, measurable three-dimensional space (Dudley p51, Passoft p9c). [8] Ideas of different authors like Arthur Eddington, Bertrand Russell and Alfred Whitehead mentioned on p108. Also mentioned on p111 of (Gardner 1962) is the following paragraph. "Later serious flaws were found in Einstein's cosmic model (General Relativity) and he was forced to abandon Mach's principle, but the principle continues to exert a strong fascination over today's cosmologists. The opposing point of view, the view that assumes a space-time metric even in the absence of stars, is really close to the old ether theory. Instead of a motionless, invisible jelly called the ether, there is a motionless, invisible space-time structure. In fact, proponents of this point of view have not hesitated to speak of rotations and accelerations as absolute". This is a good book on different ways scientists viewed relativity. [9] Newton assumed Absolute time in his calculations. (Gardner p45) Einstein used Observer time because it is the only system he thought was valid in experiments and observations. [10] The idea of fields that are responsible for different types of motion was already stated in Relativity theories. These fields carry energy, momentum etc. and have mass. In some Relativity theories fields refer to interactive wavicles such as virtual bosons and real bosons as opposed to the separate field entity proposed in this book (Brillouin p20). [11] The terms boson and fermion are established names (Apfel) presently for these wavicles but other names were used like (Dudley p88) polaron and nucleon as late as 1959. [12] External energy is a term rarely used in science presently, rather it is said that a certain amount of work results. This can be confusing as work is done in internal energy as well. [13] Einstein energy is a new term first used in this book 07-Aug-1998. [14] This is roughly a combination of the First and Second Law of Thermodynamics. It is better to combine the laws into one statement. Many versions of the Second Law of Thermodynamics only talk about devices that use a source of heat, while we know that the laws apply equally to all forms of internal energy. These laws were formulated around 1830-1850 and didn't include later knowledge from men like Gibbs and Einstein. [15] Caloric energy is a new term first used in this book 07-Aug-1998. Helmholtz energy and gibbs energy are generally regarded as chemical potentials only. Helmholtz energy is sometimes called free energy. Bound energy is not common presently, but was used by older writers, sometimes called anergy. Clausius energy is usually called exergy, but this has been common only from around 1970, sometimes called availability and essergy. [16] temperature is a new term first used in this book 07-Aug-1998. [17] (Gibbs) agrees with the modern definition of mass, but does give a good background into the history of the word mass. [18] In engineering thermodynamics, work (W) is presently defined as the ability to do useful work only, while heat (Q) is defined as the transfer of thermal energy. This definition of work creates complications when dealing with quantum physics, where work logically is done in the interactions of microscopic wavicles. [19] Based on extrapolation of Wilhelm Wien's Law of 1911 and from the fact that the photon's energy is exactly proportional to the absolute temperature. [20] Photons are not emitted and absorbed equally well at all temperatures. Different substances absorb and emit best at certain temperatures. When atoms become highly excited at high temperatures or when bombarded with large photons, the electron will absorb and discharge a photon at different definite orbit levels. A highly excited hydrogen atom will only emit 4 sizes of photons in the upper temperature region. [21] The Carnot ratio is a new term first used in this book 07-Aug-1998. It is presently accepted that entropy would be used for calculations that involve variable temperature heat transfers. Carnot believed in the caloric theory where heat was always conserved and not converted into work, it was the fall that created the work. While this made later scientists view his work as incorrect, his ideas were still valid when viewed in light of the conservation of energy. (Bailyn pg77) The fall of heat though was something that Carnot knew was converted. This fall is the same as caloric energy in this system. [22] The area under the curve is integrated to find the area and then the mean amount of the carnot ratio but this can be also calculated using natural logs. [23] Clausius thought of Entropy in 1854 but there was a delay of 11 years before introduction because of problems in dealing with internal processes (Bailyn p124) By separating out the external energy in the carnot ratio we avoid many of the difficulties with entropy. [24] Both the prism and filter experiment explained in (Goldstein p242). [25] Loschmidt mentioned in (Bailyn p254). [26] space continuum mentioned by (Herrman) and also by (Auyang). [27] Superstring theory mentioned by (Green). [28] The up to 26 dimensions of the Superstring theory mentioned by (Kaku). [29] The M-theory is mentioned as a word that replaces concepts of string and membrance theories by (Duff).
36. REFERENCES
37. REVISION HISTORY
COPYRIGHT © 1999-2023 All rights reserved |